Welcome to our in-depth exploration of density worksheet chemistry answer key, a resource designed to provide you with a comprehensive understanding of this fundamental concept. Through this detailed guide, we will delve into the intricacies of density, its formula, and its significance in chemistry.
As we embark on this journey, we will uncover the relationship between density and the properties of matter, exploring how it can be utilized to identify different substances. Additionally, we will provide a comprehensive answer key for the density worksheet, addressing common mistakes and offering detailed explanations.
Density Worksheet
Density is a measure of how tightly packed the particles of a substance are. It is calculated by dividing the mass of a substance by its volume. The formula for density is:
Density = Mass / Volume
The units of measurement for density are grams per cubic centimeter (g/cm 3) or kilograms per cubic meter (kg/m 3).
Here are some examples of how to calculate density:
- A cube of gold has a mass of 100 grams and a volume of 10 cubic centimeters. The density of gold is 100 grams / 10 cubic centimeters = 10 grams per cubic centimeter.
- A liter of water has a mass of 1 kilogram. The density of water is 1 kilogram / 1 liter = 1 kilogram per liter.
Chemistry
Density is an important property of matter. It can be used to identify different substances and to determine their purity. For example, the density of pure gold is 19.3 grams per cubic centimeter. If a sample of gold has a density of less than 19.3 grams per cubic centimeter, then it is not pure gold.
Density can also be used to determine the concentration of a solution. For example, the density of a sugar solution increases as the concentration of sugar increases.
Answer Key
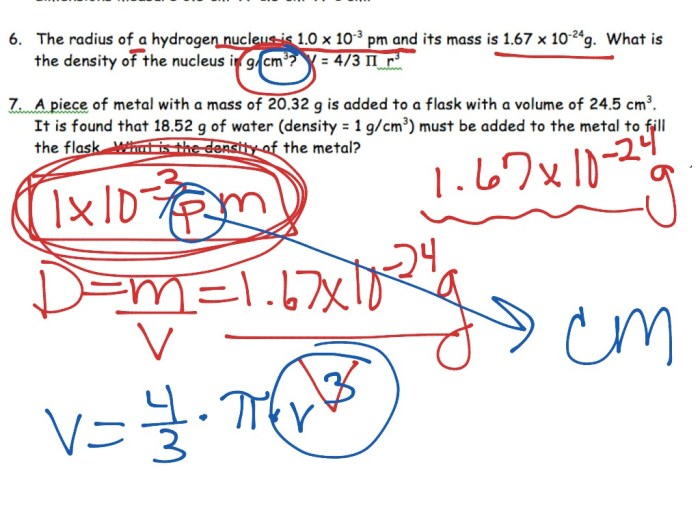
1. What is the density of a substance with a mass of 100 grams and a volume of 20 cubic centimeters?
Answer:5 grams per cubic centimeter
2. What is the volume of a substance with a density of 2 grams per cubic centimeter and a mass of 50 grams?
Answer:25 cubic centimeters
3. A sample of gold has a mass of 50 grams and a volume of 2.5 cubic centimeters. Is the sample pure gold?
Answer:Yes, the density of the sample is 50 grams / 2.5 cubic centimeters = 20 grams per cubic centimeter, which is the same as the density of pure gold.
HTML Table
| Substance | Density (g/cm3) | Units of Measurement |
|---|---|---|
| Gold | 19.3 | g/cm3 |
| Water | 1 | g/cm3 |
| Iron | 7.87 | g/cm3 |
| Aluminum | 2.7 | g/cm3 |
| Helium | 0.0001785 | g/cm3 |
Popular Questions: Density Worksheet Chemistry Answer Key
What is density?
Density is a measure of the mass of a substance per unit volume.
How is density calculated?
Density is calculated using the formula: density = mass / volume.
What are the units of measurement for density?
The most common units of measurement for density are grams per cubic centimeter (g/cm³) and kilograms per cubic meter (kg/m³).
How can density be used to identify different substances?
Different substances have different densities, so density can be used to identify them. For example, water has a density of 1 g/cm³, while gold has a density of 19.3 g/cm³.
What are some common mistakes that students make when calculating density?
Some common mistakes that students make when calculating density include using the wrong units of measurement, not converting units correctly, and making mathematical errors.