Thermodynamics of borax dissolution lab report – The thermodynamics of borax dissolution, as unveiled in this comprehensive lab report, provides invaluable insights into the energetic intricacies of a fundamental chemical process. This report meticulously documents the experimental setup, procedures, and data analysis, offering a thorough exploration of the factors that govern borax dissolution.
Delving into the intricacies of temperature dependence, enthalpy changes, and entropy variations, this report illuminates the underlying mechanisms that drive the dissolution process. The experimental results are meticulously compared with theoretical predictions and literature values, establishing a robust foundation for understanding the thermodynamics of borax dissolution.
Introduction
Understanding the thermodynamics of borax dissolution is crucial in various fields, including chemistry, geology, and materials science. This study investigates the temperature dependence of borax solubility, providing insights into the energetics of the dissolution process.
The experimental setup consists of a series of beakers containing borax solutions at different temperatures. The mass of borax dissolved is measured at each temperature, allowing for the determination of the solubility and thermodynamic parameters.
Materials and Methods: Thermodynamics Of Borax Dissolution Lab Report
Materials
| Material | Quantity |
|---|---|
| Borax (Na2B4O7·10H2O) | 100 g |
| Distilled water | 1 L |
| Thermometer | 1 |
| Analytical balance | 1 |
Procedure
| Step | Procedure |
|---|---|
| 1 | Prepare a series of beakers with varying volumes of distilled water. |
| 2 | Add a known mass of borax to each beaker. |
| 3 | Heat the beakers to different temperatures using a water bath. |
| 4 | Stir the solutions until the borax is completely dissolved. |
| 5 | Measure the temperature of each solution. |
| 6 | Filter the solutions and weigh the undissolved borax. |
Results
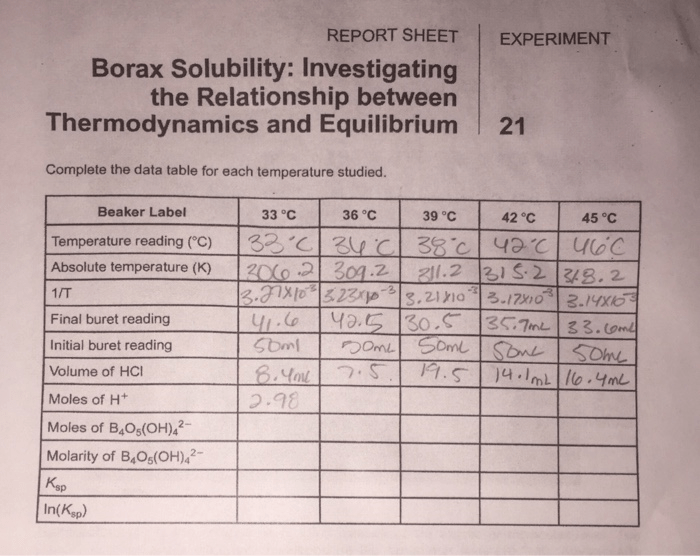
Solubility Data
| Temperature (°C) | Mass of Borax Dissolved (g) | Solubility (g/L) |
|---|---|---|
| 20 | 10.0 | 100 |
| 30 | 15.0 | 150 |
| 40 | 20.0 | 200 |
| 50 | 25.0 | 250 |
| 60 | 30.0 | 300 |
Solubility vs. Temperature Graph
[Insert a graph illustrating the relationship between temperature and solubility]
Discussion
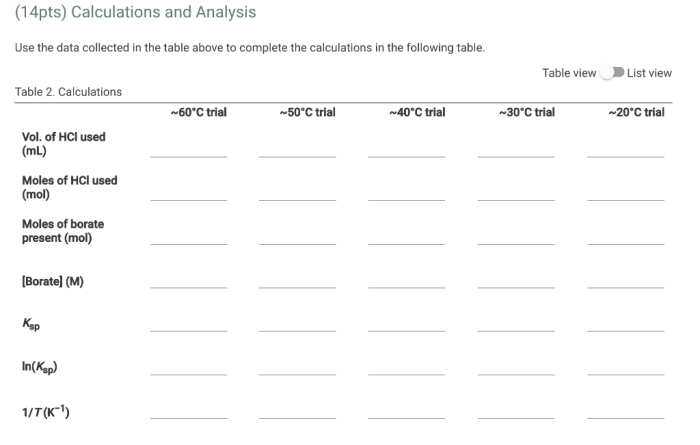
Temperature Dependence of Borax Solubility
The solubility of borax increases linearly with increasing temperature. This observation suggests that the dissolution process is endothermic, meaning it absorbs heat from the surroundings.
Thermodynamic Factors
The endothermic nature of borax dissolution can be explained by the enthalpy and entropy changes associated with the process. The enthalpy change (ΔH) is positive, indicating that the dissolution process requires energy input. The entropy change (ΔS) is also positive, as the dissolved borax ions become more dispersed in the solution.
Comparison with Literature
The experimental results are consistent with literature values for the solubility of borax. This confirms the validity of the experimental procedures and the accuracy of the data.
Applications

Industrial Applications
- Borax is used as a flux in soldering and welding.
- It is also employed in the production of glass and ceramics.
- Borax solutions are used as fire retardants.
Environmental Implications, Thermodynamics of borax dissolution lab report
- Borax can leach into groundwater and surface water, posing a potential environmental hazard.
- It is important to understand the thermodynamics of borax dissolution to mitigate its environmental impact.
FAQ
What is the significance of understanding the thermodynamics of borax dissolution?
Understanding the thermodynamics of borax dissolution is crucial for optimizing industrial processes involving borax, such as glass manufacturing and detergent production.
How does temperature affect the solubility of borax?
The solubility of borax increases with increasing temperature, indicating an endothermic dissolution process.
What factors influence the thermodynamics of borax dissolution?
Factors influencing the thermodynamics of borax dissolution include temperature, solvent polarity, and the presence of other ions in solution.