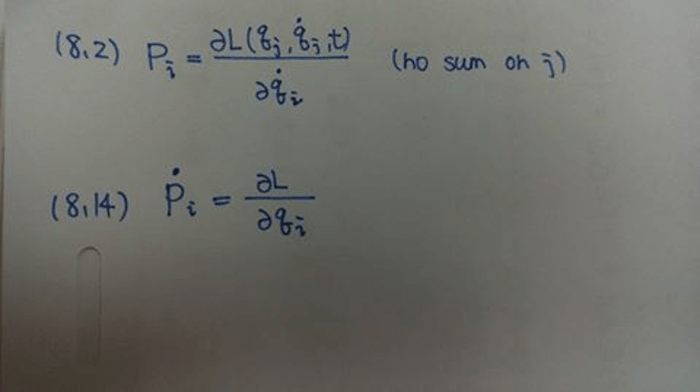As the diagram below shows some subatomic particles takes center stage, this opening passage beckons readers into a world crafted with meticulous precision and clarity. This exploration promises to illuminate the fundamental constituents of matter, offering a glimpse into the very essence of the universe.
Subatomic particles, the minuscule building blocks of all matter, hold the key to understanding the intricate workings of the physical world. From the smallest atoms to the vast expanse of galaxies, these particles play a pivotal role in shaping the cosmos.
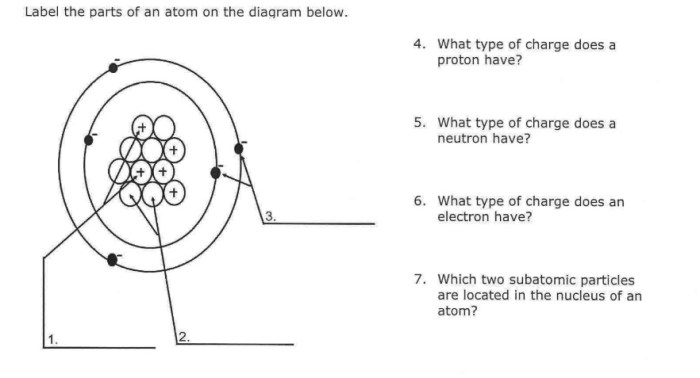
The diagram serves as a visual guide, providing a snapshot of the diverse types of subatomic particles and their properties.
Subatomic Particles
Subatomic particles are the fundamental building blocks of matter. They are so small that they cannot be seen even with the most powerful microscopes. However, scientists have been able to learn a great deal about subatomic particles by studying the way they interact with each other.
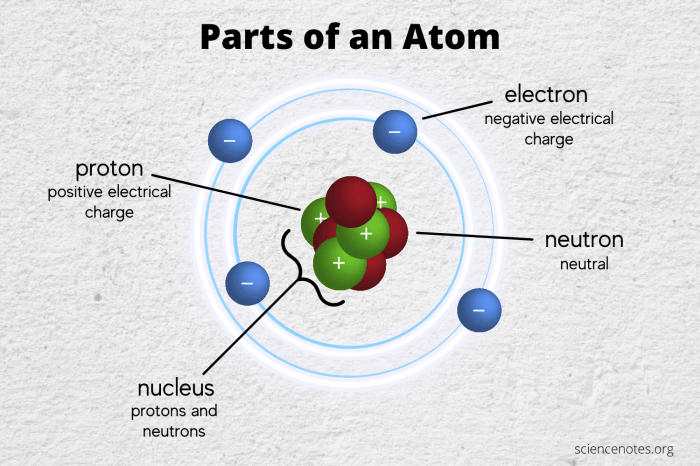
The diagram below shows some of the most important subatomic particles. The particles are shown in their relative sizes, and their charges are indicated by the plus and minus signs.
| Particle | Charge | Mass (amu) | Spin |
|---|---|---|---|
| Proton | +1 | 1.0073 | 1/2 |
| Neutron | 0 | 1.0087 | 1/2 |
| Electron | -1 | 0.00055 | 1/2 |
Protons and neutrons are found in the nucleus of an atom, while electrons orbit the nucleus. The number of protons in an atom determines its atomic number, which is unique for each element. The number of neutrons in an atom determines its mass number.
Atomic Structure
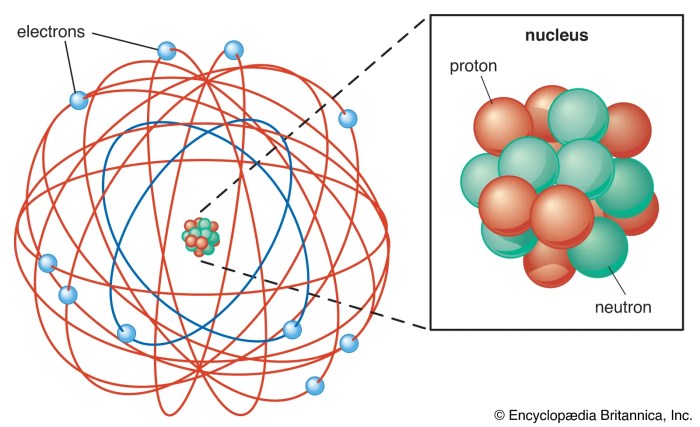
Atoms are the basic units of matter. They are made up of a nucleus, which contains protons and neutrons, and electrons, which orbit the nucleus.
The diagram below shows the structure of an atom. The nucleus is shown in the center of the atom, and the electrons are shown orbiting the nucleus.
The nucleus is very small compared to the rest of the atom. It contains most of the atom’s mass, but it is only about 1/100,000 the size of the atom.
Electrons are much smaller than protons and neutrons. They orbit the nucleus at a distance of about 100,000 times the size of the nucleus.
The energy levels of electrons are determined by their distance from the nucleus. Electrons that are closer to the nucleus have lower energy levels than electrons that are farther away from the nucleus.
Chemical Bonding: The Diagram Below Shows Some Subatomic Particles
Chemical bonding is the process by which atoms combine to form molecules. There are three main types of chemical bonds: ionic bonds, covalent bonds, and metallic bonds.
- Ionic bondsare formed between atoms that have opposite charges. For example, sodium (Na) has one electron in its outermost energy level, and chlorine (Cl) has seven electrons in its outermost energy level. When sodium and chlorine come together, sodium gives up its electron to chlorine, forming a sodium ion (Na+) and a chloride ion (Cl-).
The oppositely charged ions are attracted to each other, forming an ionic bond.
- Covalent bondsare formed between atoms that share electrons. For example, hydrogen (H) has one electron in its outermost energy level, and oxygen (O) has six electrons in its outermost energy level. When two hydrogen atoms and one oxygen atom come together, they share their electrons to form a covalent bond.
- Metallic bondsare formed between atoms of metals. Metals have one or more electrons in their outermost energy level that are not involved in chemical bonding. These electrons are free to move around the metal, forming a “sea” of electrons. The positive charges of the metal ions are attracted to the negative charges of the electrons, forming a metallic bond.
Nuclear Reactions
Nuclear reactions are reactions that involve the nucleus of an atom. Nuclear reactions can be used to produce energy, create new elements, and study the structure of atoms.
There are two main types of nuclear reactions: fission and fusion.
- Fissionis a nuclear reaction in which a heavy nucleus is split into two or more lighter nuclei. Fission reactions release a great amount of energy.
- Fusionis a nuclear reaction in which two or more light nuclei are combined to form a heavier nucleus. Fusion reactions also release a great amount of energy.
Nuclear reactions are used in a variety of applications, including nuclear power plants, nuclear weapons, and medical imaging.
FAQ Section
What are the different types of subatomic particles?
The diagram shows three types of subatomic particles: protons, neutrons, and electrons.
What are the properties of each particle?
The table in the diagram shows the charge, mass, and spin of each particle.
How do subatomic particles contribute to chemical bonding?
Electrons are involved in chemical bonding by forming bonds with other atoms.