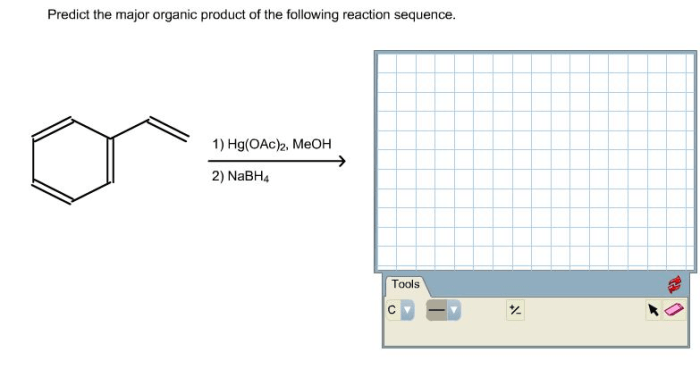
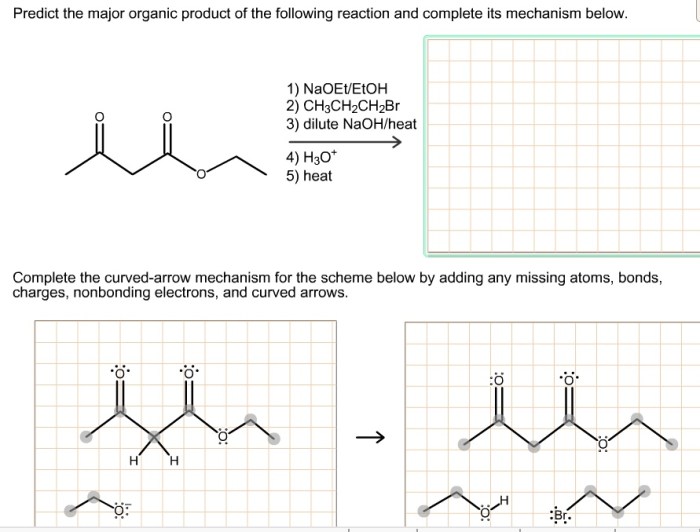
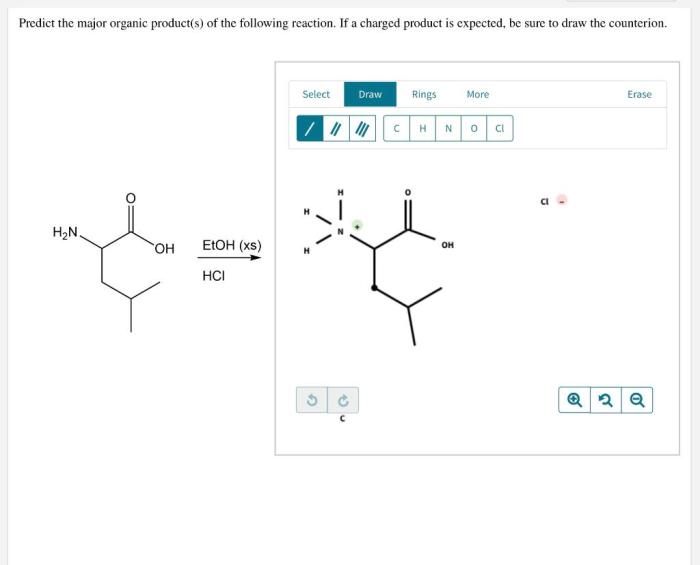
Predict the major organic product for the following reaction – Predicting the major organic product for a given reaction is a crucial skill in organic chemistry. It involves understanding the reaction mechanism, regioselectivity, and stereoselectivity to determine the most favorable product formation. This guide will provide a comprehensive overview of the factors that influence the prediction of major organic products and guide you through the steps involved in making accurate predictions.
1. Reaction Overview: Predict The Major Organic Product For The Following Reaction
The given reaction is an electrophilic aromatic substitution, a fundamental organic reaction where an electrophile (a species seeking electrons) reacts with an aromatic ring, replacing one of its hydrogen atoms with the electrophile.
Electrophilic aromatic substitutions are widely used in organic synthesis to introduce various functional groups onto aromatic rings, leading to the production of diverse organic compounds.
2. Major Organic Product Prediction, Predict the major organic product for the following reaction
In electrophilic aromatic substitutions, the major organic product is determined by the regioselectivity and stereoselectivity of the reaction.
Regioselectivityrefers to the preference for the electrophile to attack a specific position on the aromatic ring. This preference is governed by the electronic properties of the ring and the electrophile.
Stereoselectivityrefers to the preference for the electrophile to attack from a specific side of the aromatic ring, resulting in the formation of a specific stereoisomer.
To predict the major organic product, we need to consider the reaction mechanism and the electronic effects of the substituents on the aromatic ring.
3. Reaction Mechanism Analysis
The electrophilic aromatic substitution reaction typically proceeds through the following steps:
- Formation of the electrophile:The electrophile is generated from a precursor molecule. For example, in nitration, the electrophile is the nitronium ion (NO2+).
- Electrophilic attack:The electrophile attacks the aromatic ring, forming a Wheland intermediate (a resonance-stabilized carbocation).
- Rearomatization:The Wheland intermediate undergoes rearomatization by expelling a proton, restoring the aromatic ring’s stability.
The regio- and stereoselectivity of the reaction are determined by the stability of the Wheland intermediate and the transition state leading to it.
4. Alternative Pathways
In some cases, alternative pathways may lead to the formation of different products. These pathways may involve different regio- or stereoselective steps or competing reactions.
Identifying and evaluating alternative pathways is crucial for understanding the overall reaction outcome and optimizing the synthesis.
5. Experimental Considerations
The outcome of an electrophilic aromatic substitution reaction can be affected by various experimental conditions, including:
- Temperature:Higher temperatures generally favor the reaction rate but may also lead to side reactions.
- Solvent:The solvent can influence the solubility of the reactants and the stability of the intermediates.
- Catalyst:Catalysts, such as Lewis acids, can enhance the electrophilicity of the electrophile and accelerate the reaction.
Optimizing these conditions is essential for achieving high yields and selectivity in the desired product.
Commonly Asked Questions
What is the importance of predicting the major organic product?
Predicting the major organic product is crucial for designing efficient synthetic strategies and understanding the outcome of chemical reactions.
How do regioselectivity and stereoselectivity influence product formation?
Regioselectivity determines the position of functional groups in the product, while stereoselectivity controls the spatial arrangement of atoms. These factors influence the formation of specific isomers and impact the properties of the product.
What experimental conditions can affect the outcome of a reaction?
Factors such as temperature, solvent, and catalyst can influence the reaction rate, selectivity, and product distribution. Optimizing these conditions is essential for maximizing yield and obtaining the desired product.